Awesome Frozen Slime Recipe with Glitter Glue
Making Science Fun. Slime is a fun way to bring science alive. This Frozen slime recipe is a great science experiment to show how polymers work.
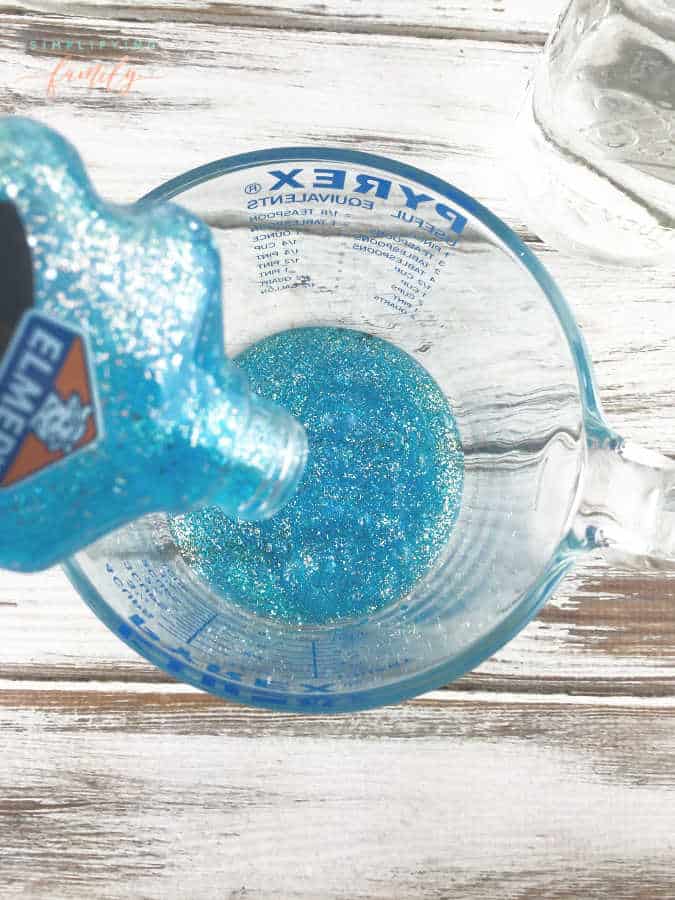
As with most children in their generation, my kids LOVE the movie Frozen. Most days “Let it Go” is sung multiple times. “Reindeer are better than people” is said throughout the day. And the first DVD was worn out.
We are now watching the Frozen Sing-Along Edition at least once a week, if not more.
But at least they love eating carrots like Sven now!
As this is our first year homeschooling, I’m trying to make learning fun.
Some days I do better than others. While I do love a good glitter craft, to say I was super excited to find glitter glue at the store was an understatement. No glitter or food dye needed.
Frozen Glitter Slime would also make a fun take-home gift from a Frozen themed Birthday Party and definitely a fun science experiment you can do at home!
How to Make Your Own Frozen Slime
What do you need to make this Frozen slime recipe? Easy!
Ingredients for Frozen Slime

borax | glitter glue | glass container | water
How to Make Glitter Glue Slime

1. Empty the contents of the glitter glue bottle into a glass container. This is a perfect job for kids to help with!
2. After the bottle is empty, fill the bottle with water and pour into the glass container with the glue.
3. Mix well.

4. After that, mix 1 teaspoon borax into 1/2 cup of warm water. (When measuring and mixing Borax, please use caution. This should be done by an adult.)
5. Mix well.

6. Slowly pour the borax/water solution in the glass container with the glue/water solution.
7. Stir to make your frozen glitter slime!

SO COOL! Super stretchy!

Easy Frozen Glitter Slime Recipe
Materials
- borax
- glitter glue
- glass container
- water
Instructions
 Empty the contents of the glitter glue bottle into a glass container. This is a perfect job for kids to help with!
Empty the contents of the glitter glue bottle into a glass container. This is a perfect job for kids to help with! 
- After the bottle is empty, fill the bottle with water and pour into the glass container with the glue.
- Mix well.

- After that, mix 1 teaspoon borax into 1/2 cup of warm water. (When measuring and mixing Borax, please use caution. This should be done by an adult.)

- Mix well.
- Slowly pour the borax/water solution in the glass container with the glue/water solution.

- Stir and watch the magic happen.

Notes
Frozen Slime Recipe Science Lesson
This is a lesson in polymers. Polymers are made out of long strands of molecules, similar to a beaded necklace.
Glue contains an ingredient called polyvinyl acetate, which is a liquid polymer. Borax helps the polymer strands stick together, creating slime. Pretty awesome!
You can make bouncy balls, pull and stretch the slime, such a fun science experiment.
So much fun!
Frozen Slime Recipe Polymers Science Experiment
This is a polymers science experiment for kids. Polymers are made out of long strands of molecules, similar to a beaded necklace.

Glue contains an ingredient called polyvinyl acetate, which is a liquid polymer. Borax helps the polymer strands stick together, creating slime. Pretty awesome!
You can make bouncy balls, pull and stretch the slime, such a fun science experiment.

The kids have LOVED playing with the blue frozen slime recipe. Next time I’m going to try this with white glue and see if it makes a difference.
Have you made “Frozen” slime?
What did you think? Did your kids love it?
Want another Princess Slime recipe, try this Princess Slime Recipe!
Disney Magic at Home
Bringing Disney Magic Home























Our granddaughter LOVES Frozen. She dances to “Let It Go” all the time. Talk about great exercising!
For the longest time, we didn’t have snow. We made Sven with the following items: Three cucumber slices for the snowballs, a mini sweet pepper for the hat, celery sticks for the arms, sliver of carrot for the nose, pecans for the eyes and buttons. Make a Sven out of veggies….so much fun!
Thanks for the frozen slime idea.
Definitely! We love impromptu dance parties!! I LOVE that idea. A veggie snowman!! That’s one way to get kids to get their veggies.
So fun!! I can’t wait to try this one with my kids.
Oh yes! My kid will love this!
Can’t wait to try this with my 10 year old son. He’s going to love it!
I do this activity with 60+ kids every year for a tour of a plastics company. Everyone loves it. I’ve not done it with glitter glue… It generally doesn’t come in gallon jug. The white works fine. I’ve even added food coloring.
how do you store this & how long does this last? Would like to know how far in advance I can make it for a class.
It does dry out. I kept ours in the measuring cup at room temperature for about 3 days. I stuck it in the refrigerator to see what it would do. It’s less like slime and more like a hard gel after about a week. Still fun though :). I’m going to try it with 1/2 a teaspoon of Borax and see if it stays like slime longer.
Hi what can I use instead of borax?We don’t have borax .
I’ve seen contact lens solution and liquid starch.
Can you do it with superglue instead of normal glue?
No. Please use regular glue.
I think its cool and im thinking about doing it.